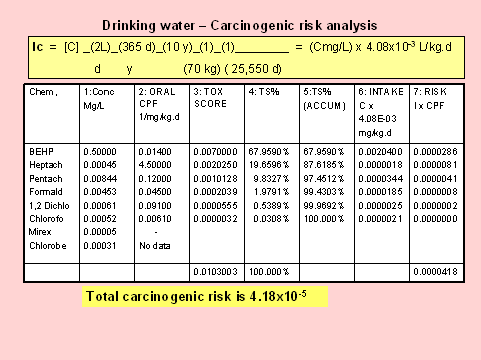
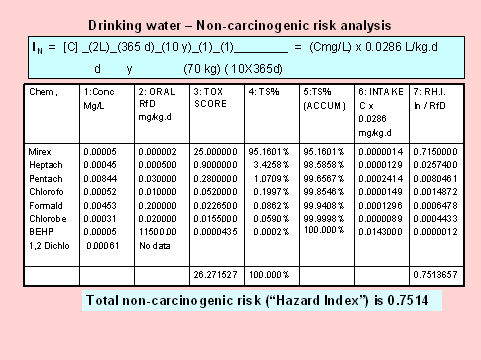
RISK ASSESSMENT AND COMMUNICATION [Chronic risk: part 2]
|
The following are a collection of 17 revised examples of the calculations of intakes, chronic risks, and hazard indices using the EPA four-step model. They are displayed with the problem, the assumption used, & the solutions. |
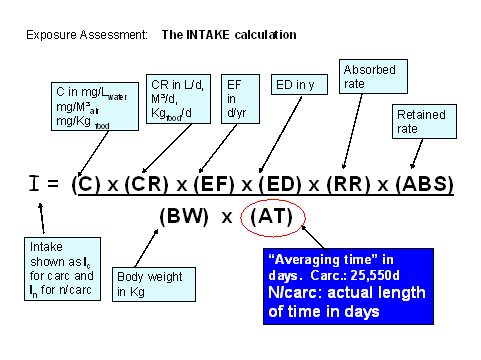
Reminder of the INTAKE calculation......
AS A HELP IN ENCOURAGING YOU TO WORK OUT THE SOLUTIONS YOURSELF AND NOT JUST TO RELY ON THE ILLUSTRATED EXAMPLES, I HAVE TEMPORARILY MASKED SOME OF THE SOLUTIONS IN THE FOLLOWING 17 CHALLENGES. AFTER YOU TRY THE CALCULATIONS AGAIN, YOU CAN REVEAL THE SOLUTION BY PULLING YOUR CURSOR OVER THE 'EMPTY' CELL.
|
1. Dichlorvos exposure in a teacherWhat is your estimated lifetime cancer risk for an adult individual who is exposed to drinking water while at home containing 0.001 mg/L dichlorvos. The exposure is for 2 years. She is employed as a grade 3 teacher during that time at a school not affected by the contaminated water. |
|
From the question, you have these values C = 0.001mg/L ED = 2 y Other values and assumptions : CR = 2L/d (standard tables) BW = 70 kg ( " ) AT = (carc.) 25550d ( " ) EF = days-equivalents exposed per year = full year MINUS work time = 365d MINUS (9 months x 20d/m x 8h/24h) = 365d — 60d = 305d RR = 1 (default) ABS = 1 ( " ) |
SOLUTION: Ic = ( 0.001 mg/L ) (2 L/d) (302 d/y) (2y) 70kg 25,550d = 6.75 x 10-7 mg / Kg.d Risk = 6.75 x 10-7 mg / Kg.d x 0.29 (mg / Kg.d)-1 = 1.95 x 10-7 This is less than then the de minimis level of 1 in a million and is probably acceptable by the community (although special considerations and precautions are appropriate for children, infants and the unborn).
|
|
2. Mine-worker / nickel dust You are assisting in the assessment of a worker in a Nickel mine. The mean concentration of nickel refinery dust in air is 2.0 microgram/M³. The workers are at work about 200 days a year in single 8 hr shifts. They have been at this work for 8 years. Has the typical worker been exposed to excessive nickel? Calculate the risk of additional risk of death from cancer. What recommendations, if any, would you make to the company to reduce the risk for future workers? |
|
Assumptions and values used (NOTE: These values would normally be given to you) [C] = 2 microgram/M³. or 0.002 mg/M³. CR = 1.2 M³ /Hr (note this is in hours and must show days so x(24h/d) EF = 8 hr shift per day, x (8h/24h) x (200 d/yr) ED = 8 y BW = 70 Kg AT = 25,550 d ABS: 1 RR: 1 |
|
SOLUTION Ic = (0.002 mg/M³) x (1.2 M³ /h) x (24h/d) x (8h/24h) x (200 d/y) x (8 y) 70 Kg x 25,550 d
Ic = 0.0000172 mg/Kg.d
RISK = 0.0000172 mg/Kg.d x 0.84 = 0.00001443 which is more than 14 times the de minimis level. Recommendation: reduce the air concentration to 1/14 the current [Conc] (i.e. to 0.00014 mg/M³ or 0.14 micrograms/M³)
|
|
3. Carrots and Copper Cyanide (different
calculation) (A) Find intake in mg/Kg.d for copper cyanide in carrots detected at rate of 4 mg/Kg carrots. Assess for 9 y/o child, with daily consumption of 100 g carrots per day, for her lifetime to date. If these carrots are to be consumed in future, what would you recommend (B) as the maximum intake of carrots a day assuming the concentration of copper cyanide remains the same? (C) as the maximum concentration of copper cyanide in the carrots assuming the consumption remains at 100 g per day? |
|
Assumptions and values used (NOTE: These values would normally be given to you) C: 4 mg /Kg(food) 9 yr old child CR: 100g carrots/d EF: daily ED: 9 yr AT: (365x9) d BW: 29 kg ABS : assume 1.0 RR: assume 1.0 |
|
Calculation (A) IN = (4mg/Kgf) x (0.1Kgf/d) x (365d/y) x (9y) x (1) x (1) 29 Kg bw x (365 x 9) d
This can be simplified because the EFxED cancel the AT
IN = (4mg/Kgf) x (0.1Kgf/d) 29 Kgbw
= 1.38 x 10-2 mg/Kg.d
H.Index : IN mg/Kg.d / RfD 1.38E-02 mg/Kg.d / 5.00E-03 mg/Kg.d = 2.76 - which is nearly 3 times higher than the "action level" generally considered for a non-carcinogen. (B) Assuming [C] in carrots remains constant at 4mg/Kg, the daily intake would need to be reduced to 100g / 2.76 or 36 g a day (C) Assuming she must continue to eat 100g carrots a day, the [C] in carrots would need to be reduced to 4mg / 2.76 = 1.45 mg/Kg
|
4. Water contamination: Mining townThe water in a mining town has been found to be contaminated with metal salts and chemicals associated with assaying and extraction of ores. Carry out an assessment of any carcinogenic and non-carcinogenic effects arising from an adult resident's lifetime of water consumption. The following are the concentrations recorded.zinc cyanide 0.04 mg/Lzinc phosphide 0.02 mg/Lnickel (soluble) salts 0.23 mg/Lmanganese 0.19 mg/L |
|
Assumptions and values used
(NOTE: These values would normally be given to you) EF: 365d/y ED: 70y BW: 70Kg Water consumpt: 2 L/d Also assume that as the assessment is over a lifetime, the AT rates and EDxEF rates are equivalent. |
|
Solution:
The intake calculation:
In
= [Cmg/L] x 2L/d x 1/70Kg =
[Cmg/L] x
0.0286 Solution: Creating the assessment. .... There are no CPF data shown for any of these chemicals. Therefore there is no carcinogenic assessment possible. The following is the non-carc. assessment leading to Hax.Index. |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| CONC | RfD | TS | TS% | TS%ACCUM | INTAKE | H.Index | |
|
MG/L |
Cx(0.0286) |
||||||
| zinc phosphide | 0.02 | 3.00E-04 | 66.67 | 68.05% | 68.05% | 0.000572 | 1.907 |
| manganese | 0.19 | 1.00E-02 | 19.00 | 19.39% | 87.44% | 0.005434 | 0.543 |
| nickel (sol) salts | 0.23 | 2.00E-02 | 11.50 | 11.74% | 99.18% | 0.006578 | 0.329 |
| zinc cyanide | 0.04 | 5.00E-02 | 0.80 | 0.82% | 100.00% | 0.001144 | 0.023 |
| 9.80E+01 | 100.00% | 2.802 |
|
5. Ethyl Ether in water
Estimate (A) the intake for 0.02 mg/L ethyl ether in drinking water and (B) the expected health outcomes for sedentary male at residence for 1 year |
|
Assumptions and values used. (NOTE: These values would normally be given to you) [C] 0.02 mg/L (a non-carcinogen) Assume 365 d/y, 2L/d water cons, ABS and RR: 100% adult bw: 70 Kg. AT: 365 d ED: 1 y EF: 365d/y |
|
Solution (A)
In = (0.02 mg/L) (2L/d) (365d/y) (1y) (1) (1) (70 Kg) (365 d)
Because EDxED are the same as AT, we can simplify to
In = (0.02 mg/L) (2L/d) = 5.714E-04 mg/Kg.d (70 Kg)
(B) The HI is calculated as In / RfD 5.714E-04 mg/Kg.d / 2E-01 mg/Kg.d
= 0.0028 (much below the level at which concern might be expected or expressed)
|
|
6. Airborne Solvent Calculate the intake for an adult's 5-year occupational exposure to an airborne carcinogenic solvent. |
|
Assumptions and values used (NOTE: These values would normally be given to you)
An 8-hr working day for 200 working days a year. ED: 5 y The work is light, so respiration of 1.2 m3/h, 70% RR and 100% Abs |
|
Solution
Ic = [C] x [CR] x EF x ED x RR x ABS BW x AT
= (1.2 m3/h) (8h/d) (200 d/y) (5 y) (0.7) (1) (70 Kg) (25550d)
= [C] x 0.00376 m3/Kg.d
|
|
7 Child: local airborne non-Carc. Calculate the intake for a 20 Kg child of a non-c continuously emitted from a chemical plant near the residence for one year. |
|
Assumptions and values used: (NOTE: These values would normally be given to you). 20Kgbw (see table on p37) suggests 6yr old, with resp. rate about 0.35 cu M per day. EFxED: 9 months x 20 d/mo x 6.5 h/d. 100% abs, and RR. AT: 10 months at 30.5 d/mo |
|
Solutions IN = [C] (CR) (EF) (ED) (RR) (ABS) (BW) (AT)
= [C] (0.35 m3/h) (6.5h/d) (180d/y) (1 y) (1) (1) (20 Kg) (305d) = [C] x 0.0671 m3/Kg.d
. Note that any slight variation of this type would be virtually INVISIBLE in the final outcome. when we are concerned with orders of magnitude ( 10x, 100x, 10-3, 10-4, etc.), something like the difference between 0.32 and 0.34 is of no importance at all. |
|
8. gamma-Hexachlorocyclohexane
(γ-HCH) in water Concentration of HCH in drinking water at 0.7 mg/L Estimate intake for adult resident (exposed during hours of 5pm-8am each day. ED: 18 months. γ-HCH is non-Carc. only. Also calc H.I. If H.I >1, calculate water concentration which would maintain HI<1 |
|
Assumptions and values used
(NOTE: These values would normally be given to you) EF: (15h/24h) (365d/y) ED: 1.5 y AT: 1.5x365 d CR: 2 L/d |
|
Solution Intake (N) = 0.7 mg.L x 2 L/d x 15h/24h x 365 d/y x 1.5 y 70 Kg (1.5 x 365)d
simplified to 0.7 mg.L x 2 L/d x 15/24
x
70 Kg x = 0.0125 mg/Kg.d H.I. = ( 0.0125 mg/Kg.d) / (3.00E-04) = 41.67 To maintain H.I. <1, water concentration must be (0.7 mg/L) x 1/42 = 0.0167 mg/L
|
|
9. 1-2 Dichloroethane in air
(adjusted April 5: the calculation should be done as a carcinogen.
Therefore the AT value should have been 70x365d) Calculate intake for adult (assumed sedentary) daily (8 hr shiftwork) for 2 years
|
|
Assumptions and values used (NOTE: These values would normally be given to you) [C] : 0.002 mg/m3 EF: (8h/24h) x (200d/y) ED: 2 y AT (70x365)d air breathed: assume sedentary : 0.85 m3/h
|
|
Solution Ic = [0.002mg/m3] x (0.85 m3/h x 24 h/d) x (8h/24h) x (200d/y) x 2y x 1 x 1 70 Kg x (365 x 70)d Ic = 3.042 x 10-6 mg/kg.d risk= Ic x SF = 3.042 x 10-6 mg/kg.d x 9.10E-2 (mg/kg.d)-1 = 2.768E-07 which is close to, but less than, the de minimis level.
|
|
10
PCP in lumber mill workers (previously #7) Workers in a lumber mill have an estimated daily intake (per person) of pentachlorophenol (PCP) through food and water pathways combined of 0.11 mg. Inhalation of PCP through airborne vapour adds another 0.04 mg per person per day. What is the incremental cancer risk for these workers from these combined exposures?
|
|
Assumptions and values used.
(Note that normally you would be given these values) ED: working life of 50 y EF: 200 d/y Adult bw: 70 Kg AT (carc): 25,550 d Notes: (1) that the INTAKE has already been calculated BUT as "per person, per day". and (2) that whereas normally the different pathways (ingestion and inhalation) would be separate calculations because they are subject to different SF/CPF, in this case we only have a single SF/CPF, so we can combine to a single intake and single calculation. The problem is that an inhalation CPF is not given and instead the oral CPF is being used. This can be contentious and may not always be acceptable. We would not give you a situation such as this on an exam.
|
|
Solution:
Intake per person = 0.15 mg /d We can assume the time factors (ED, EF, AT) have similarly already been considered iin calculating the intake But we need to calculate the intake as mg/Kg.d = _0.15 mg / d_ = 0.002143 mg / Kg.d 70 Kg The increased risk is Intake x SF = (2.143E-03 mg/Kg.d) x 1.20E-01 (mg/Kg.d)-1 = 0.0002572, which is 257 times the de minimis level
|
|
11
Heptachlor and potatoes (previously #8) A group of subsistence farmers grow potatoes on their land. They and their families eat up to 0.7 Kg potatoes The soil has been found to contain a residual contamination with heptachlor (at 0.05 mg/Kg soil). Water from the site's shallow wells is also found to contain heptachlor (0.004 mg/L). Calculate the incremental cancer risk and the hazard index for one year's exposure.
|
| Assumptions and values used:
CR: 0.7Kg potatoes/person/day EF: 365 d/y EF: 1 y Bioconcentration factor potatoes/soil : 2.5 The intake calculation is performed without the EDxEF or AT. Of course this would be normal where the exposure is a "24/7" lifetime exposure and the numerator cancels the denominator. But in this case, the exposure is ONE year so EDxEF should be 1y x 365d/y, but the AT should still be 25,550d
|
Solution:
we calculate the the two pathways separately and
combine the intakes (equivalent to column 6)
(FOOD) Ic = 0.05mg/Kgf x 2.5 x 0.7 Kgfd x 365d x 1 = 1.79E-0570Kgbw x 25,550d
(WATER) Ic = 0.004 mg/L x 2L/d x 365 d = 1.63E-0670Kg x 25,550d(COMBINED) (1.79E-05 + 1.63E-06) mg/Kg.d = 1.95E-05 mg/Kg.dThe risk is calculated at I x SF 1.95E-05 mg/Kg.d x 4.5E+00 = 8.79E-05 which is 88 times higher than de minimis
|
| (The equivalent calculation will yield the H.I.) |
| 12 Hydrazine Sulfate: If the "de minimis" level is accepted as the "background" level for the population of 150,000 persons, how many additional cases of cancer would you expect if each person were to be exposed to 0.002 mg hydrazine per day on a continuing basis? |
|
Assumptions and values
used: Note that the intake has already been calculated! Also note that the intake is given as mg/person per day. In other words we still need to get it into the form we use, which is mg/Kg.d Ic = per person intake divided by average bw of 70 Kg: (0.002 mg/d) / (70kg) = 0.0000285 mg/kg.d It remains only to multiply the Ic by the SF of 3.0 (having confirmed first that hydrazine is a carcinogen) Assume every day + all day for lifetime, so EDxEF cancel with AT
|
|
NOTE:THIS ITEM IS UNDER REVIEW; PLEASE CHECK
BACK IN 24 HRS Solution: Risk = 2.85 x 10-4 mg/kg.d x 3.0 (mg/kg.d)-1 = 8.55 x 10-5 This is of course MORE than de minimis (by 85.5 times) The question of how many additional cases would ensue from the population's exposure can be answered by recognizing that this risk (8.55 x 10-5 ) is for any ONE person. Therefore we multiply ( 8.55 x 10-5 ) by 150,000 to obtain 12.8 (or 13) additional deaths from cancers. |
| 13 Any airborne carcinogenic exposure Calculate the intake for an adult's 5 year occupational exposure to an airborne carcinogenic solvent during an 8 hr working day for 200 days per year. The work is fairly light, so assume respiration at 1.2 m3/hr, with 70% retention and 100% absorption. (Note that this calls for the intake calculation to be determined without the concentration being included. In this way the value can be used for exposure to ANY airborne carcinogens materials which are in the same path and have the same characteristics) |
|
Assumptions and values used.
C: (not used) CR: (1.2 m3/hr) (given) EF: 200 d/y (given) ED: 5 y (given) ABS: 1.0 (given) RR: 0.7 (given) BW: 70kg (from standard values) AT: 25,550 d (from standard values) |
|
Solutions: Ic = [C mg/m3] x (1.2 m3/h) (24h/d) (8h/24h) (200d/y) (5y) (1.0) (0.7) ( 70kg ) ( 25 550d ) = [C mg/m3] x (0.000376 m3/kg.d) This value would be used in column 6 to calculate the intake values for a list of substances
|
|
14. Any non-carcinogen intake
value for a 20 Kg child Calculate the intake for a
20 Kg child for a non-carcinogen continuously emmitted from a chemical plant
near the residence for six months.
Assume all living and recreation, school, etc. is in ther asame area. Assume 100 abs and 100 retention. |
|
Assumptions and values used C: not used CR: 0.35m3/h (20 kg child is probably about 6 yrs old (from standard values table) EF: 365 d/y (given) ED: 0.5 yr (given) Abs = RR = 1.0 (given) BW= 20 kg (given) AT = days in 0.5 y = 183d |
| In = [C] x (0.35 m3/h) (24h/d) (365d/y) (0.5 y) (1) (1) ( 20 kg ) ( 183 d ) Note that the EDxED cancel the AT because it is full time exposure. So we can simplify using In = [C mg/m3] x (0.35 m3/h) (24h/d) ( 20 kg ) giving [Cmg/m3] x 0.42 m3/K.d
|
| 15: Long-term exposure to non-carcinogen: population of all ages. A town's drinking water has been found to have been contaminated by a chemical dump that was buried and forgotten in 1930. The first chemical of interest is phenol, a non-carcinogen The water contains 230 ppb phenol and the assessment will assume a constant level of exposure at this level for the past 78 years. Assess the health status to this exposure up to the present date. The the ground water has this year been replaced by a municipal supply. |
|
Assumptions and Values used.
Some people may have been exposed for their whole life if they were present
continually since 1930. Others may have been born in the community
only this year. The best approach is to take an "average" exposure
duration therefore of 30 years (roughly half a lifetime, and allowing for
the community to have grown and other people not to have been present the
whole time). The AT would cancel out the EDxEF.
C: 0.23 mg/L (note the original C was 230 ppb or 230 micrograms/L CR: 2 L/d EF: 365d/y ED: 30 y 70 kg AT: 30 x 365 d
|
|
Solution Ic = ( 0.23 mg/L) (2 L/d) (365 d/y) (30 y) ( 70 kg ) ( 365 d/y x 30 y) simplifies to Ic = ( 0.023 mg/L) (2 L/d) = 0.00657 mg/Kg.d ( 70 kg ) The H.I. becomes: In / RfD = (0.00657) / (0.60) = 0.011 This is far below any action level (1.0), and should probably be ignored, Note that the elimination of the ED, EF and AT reduces the calculation to a constant hazard value for any period of time. Compare this with the following (carcinogen) example.
|
|
16
Long-term exposure to
carcinogen: population of all ages.
The same water supply (see situation #16) contained
benzyl chloride at a concentration of 100 ppb. Use the same parameters to
estimate the carcinogenic risk
|
|
Assumptions and values used: In this case, the mid-range of the ED (30
years) can be used but the AT must remain 70x365d. C: 0.100 mg/L , a carcinogen. (note the original C was quoted as 100 ppb or 100 micrograms/L CR: 2 L/d EF: 365d/y ED: 30 y 70 kg AT: 70 x 365 d
|
|
Solution: Ic = ( 0.100 mg/L) (2 L/d) (365 d/y) (30 y) ( 70 kg ) ( 25,550d)
= 0.0012 mg/kg.d
The risk is therefore 0.0012 mg/kg.d x 0.17 (mg/kg.d)-1 = 2.03 E-4
This exceeds the de minimis level by two hundred times.
|
|
17
1,2-Diphenylhydrazine is being released during the daytime from a
refinery in the concentration of 12 ppb air. The exposure may have been
constant for a year and a half. Calculate the health effects of such
exposure for a sedentary typical adult who may have been permanently
resident in this area for that time.
|
|
Assumptions and values used [C}: 12 ppb = 12 micro g / m3 = 0.012 mg/m3 1,2-Diphenylhydrazine vapour in air CR: (0.85 m3/h) air EF: 365d/y ED: 1.5y BW: 70kg AT: 25550 d (carcinogen) |
|
Solution: Ic = (0.012 mg/m3) ( 0.85m3/h) (24h/d) ( 365 d/y) (1/5y) (70kg) (25550 d) = 7.49E-05 mg/kg.d The risk estimate is therefore Ic x SF or 7.49E-05 x 0.8 = 5.99E-05 This is 60 times greater than the de minimis level, and should probably receive some prompt corrective action
|
Another example of the composite calculation.